Articles from NorthStar Medical Radioisotopes, LLC

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced the appointment of Victor Miller as Chief Financial Officer, effective December 1st 2025.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · December 16, 2025

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced the appointment of Kathy Spencer-Pike as Chief Commercial Officer, effective December 1st 2025.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · December 16, 2025

NorthStar Medical Radioisotopes, LLC (NorthStar), a leading radiopharmaceutical company, is proud to announce the formation of a multi-faceted strategic partnership with the University of Wisconsin School of Medicine and Public Health, working closely with the school’s Initiative for Theranostics and Particle Therapy (ITPT) to advance research and support workforce development in the nuclear medicine sector. This collaboration represents a significant advancement in efforts to create closer ties between industry and academia in order to foster innovation and further strengthen the nuclear medicine development in Wisconsin.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · September 23, 2025

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and Ariceum Therapeutics, a private biotech company developing radiopharmaceutical products for the diagnosis and treatment of certain hard-to-treat cancers, today announced the signing of a supply agreement for the therapeutic medical radioisotope, actinium-225 (Ac-225).
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · December 11, 2024

NorthStar Medical Radioisotopes, LLC, and PeptiDream Inc. (President: Patrick C. Reid, Tokyo: 4587) today announced that PeptiDream’s wholly owned subsidiary PDRadiopharma Inc. (President: Masato Murakami) has entered into a strategic collaboration with NorthStar, a global innovator in the development, production, and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging. PDRadiopharma is the leading radiopharmaceutical company in Japan, delivering high-quality radiodiagnostic imaging agents for use in SPECT and PET scans across Japan for more than 50 years, and now as a part of PeptiDream, is focused on bringing to patients the next-generation of targeted radiodiagnostics and radiotherapeutics for the diagnosis and treatment of a broad range of cancers.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · December 5, 2024

NorthStar Medical Radioisotopes, LLC today announced the signing of a strategic Master Supply Agreement with Cellectar Biosciences, Inc. (NASDAQ: CLRB) under which Cellectar will acquire and integrate NorthStar’s n.c.a. Ac-225 into Cellectar’s proprietary Phospholipid Ethers (PLE) delivery platform to expand the platform’s capability to produce a diverse range of radiotherapeutic molecules.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · November 21, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging hosted a grand opening event and ribbon-cutting at the state-of-the-art NorthStar Dose Manufacturing Center in Beloit, Wis., on October 3, 2024. Guest speakers joined with other industry and healthcare leaders, NorthStar customers, suppliers, and partners to share perspectives on the exciting growth of nuclear medicine, the need for reliable U.S. radioisotope supply to support better patient outcomes and how NorthStar is critical to this success.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · October 22, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production, and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced it will participate in Oppenheimer & Co. Inc.’s Targeted Radiopharmaceutical Therapies in Oncology Summit being held in New York City on Tuesday, October 8th, 2024. The meeting, Oppenheimer’s second annual event focused on issues, innovations and trends related to development and use of radiopharmaceuticals for treatment of cancer, will consist of topical panels, company presentations, opportunity for various one-on-one meetings and networking.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · October 3, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production, and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and Convergent Therapeutics, Inc., a clinical stage radiopharmaceutical company, today announced the signing of a strategic contract manufacturing services agreement. Convergent’s lead asset CONV01-α is a prostate-specific membrane antigen (PSMA)-targeted monoclonal antibody linked to Ac-225 and is currently being investigated as a treatment for prostate cancer.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · August 13, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in development, production and commercialization of radiopharmaceuticals used to detect and treat cancer and other serious diseases, today announced that it has entered into a sponsorship agreement with an undisclosed major U.S. research institution which will undertake an investigator-initiated research project entitled “Program to Find a Cure for ANCA-associated Vasculitis Through Research”.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · July 30, 2024

NorthStar Medical Radioisotopes, LLC (NorthStar) and BWXT Medical Ltd., a subsidiary of BWX Technologies, Inc. (NYSE: BWXT) today announced that they have signed a Master Services Agreement (MSA), which will facilitate the production of actinium-225 (Ac-225), a critical medical isotope used to kill cancer cells while minimizing the impact to healthy tissues.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · July 22, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used to detect and treat cancer and other serious diseases, today announced that it has completed and will commission a new cleanroom facility on its campus in Beloit, Wis. to support clinical trials. The completion, qualification and opening of the new cleanroom to support both current clinical trials and full-scale manufacturing that will commence in July of this year represents a key milestone in NorthStar’s effort to help accelerate and expand access to game-changing radiopharmaceuticals for patients around the world.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · June 4, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in development, production and commercialization of radiopharmaceuticals used to detect and treat cancer and other serious diseases, today announced the signing of a Clinical Supply Agreement with Clarity Pharmaceuticals for the production of 67Cu-SAR-bisPSMA drug product for Clarity’s Phase I/II and Phase III trials. The overarching Master Service agreement and associated Clinical Supply Agreement are effective immediately and the initial production of supply to support Clarity trials is expected to occur before the end of calendar 2024.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · April 10, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production, and commercialization of radiopharmaceuticals used to detect and treat cancer and other serious diseases, today announced that Frank Scholz, Ph.D., President and Chief Executive Officer will present at the upcoming 2024 Jefferies Radiopharma Innovation Summit.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · April 4, 2024

NorthStar Medical Radioisotopes, LLC (“NorthStar”), a global innovator in development, production and commercialization of radiopharmaceuticals used to detect and treat cancer and other serious diseases, and Alpha-9 Oncology Inc. (“Alpha-9”), a clinical stage biotechnology company developing differentiated and highly targeted radiopharmaceuticals for treatment of a range of cancers, today announced a long-term strategic supply agreement for NorthStar to provide Alpha-9 with its non-carrier added (n.c.a.) Ac-225 for use in its development and clinical programs.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · January 8, 2024

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used to detect and treat cancer, today announced that it will participate in the upcoming 2023 Truist Securities Biopharma Symposium being held November 8 to 9, 2023, in New York, NY. NorthStar executives Stephen Merrick, Executive Chairman, and Paul Estrem, Executive Vice President and Chief Financial Officer, will host one-on-one meetings with investors on November 8, 2023.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · November 6, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals, and Curadh MTR Inc. (Curadh), a global MTR focused clinical, research and advisory organization, today announced the signing of a strategic long term supply agreement for the therapeutic radioisotope non-carrier added (n.c.a.) actinium-225 (Ac-225).
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · August 29, 2023

NorthStar Medical Radioisotopes, LLC (“NorthStar"), a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, is pleased to announce that the first patient has been dosed in a clinical trial using NorthStar’s electron accelerator-produced Cu-67. Administration of the therapeutic dose took place as part of an ongoing Phase I/IIa theranostic clinical trial conducted by Clarity Pharmaceuticals to investigate the safety and efficacy of Cu-67 SARTATE in pediatric patients with high-risk neuroblastoma. The event marks a key milestone in the exclusive Cu-67 supply agreement between both companies.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · August 28, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced the signing of a supply agreement for the therapeutic medical radioisotope, actinium-225 (Ac-225) with Bayer. Under the terms of the agreement, NorthStar will provide Bayer with its environmentally preferred, non-carrier added (n.c.a.) Ac-225. NorthStar’s Ac-225 will be used by Bayer for several of its radiopharmaceutical programs.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · July 18, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and Nucleus RadioPharma, a full-service Contract Development and Manufacturing Organization (CDMO) dedicated to building robust and reliable clinical and commercial supply chains for targeted radiotherapies, today announced the signing of a supply agreement for the therapeutic radioisotope actinium-225 (Ac-225). Under terms of the agreement, NorthStar will supply its high purity, non-carrier added (n.c.a.) Ac-225 to Nucleus. Nucleus will use NorthStar’s Ac-225 for their customers’ radioligand pharmaceutical programs.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · June 27, 2023
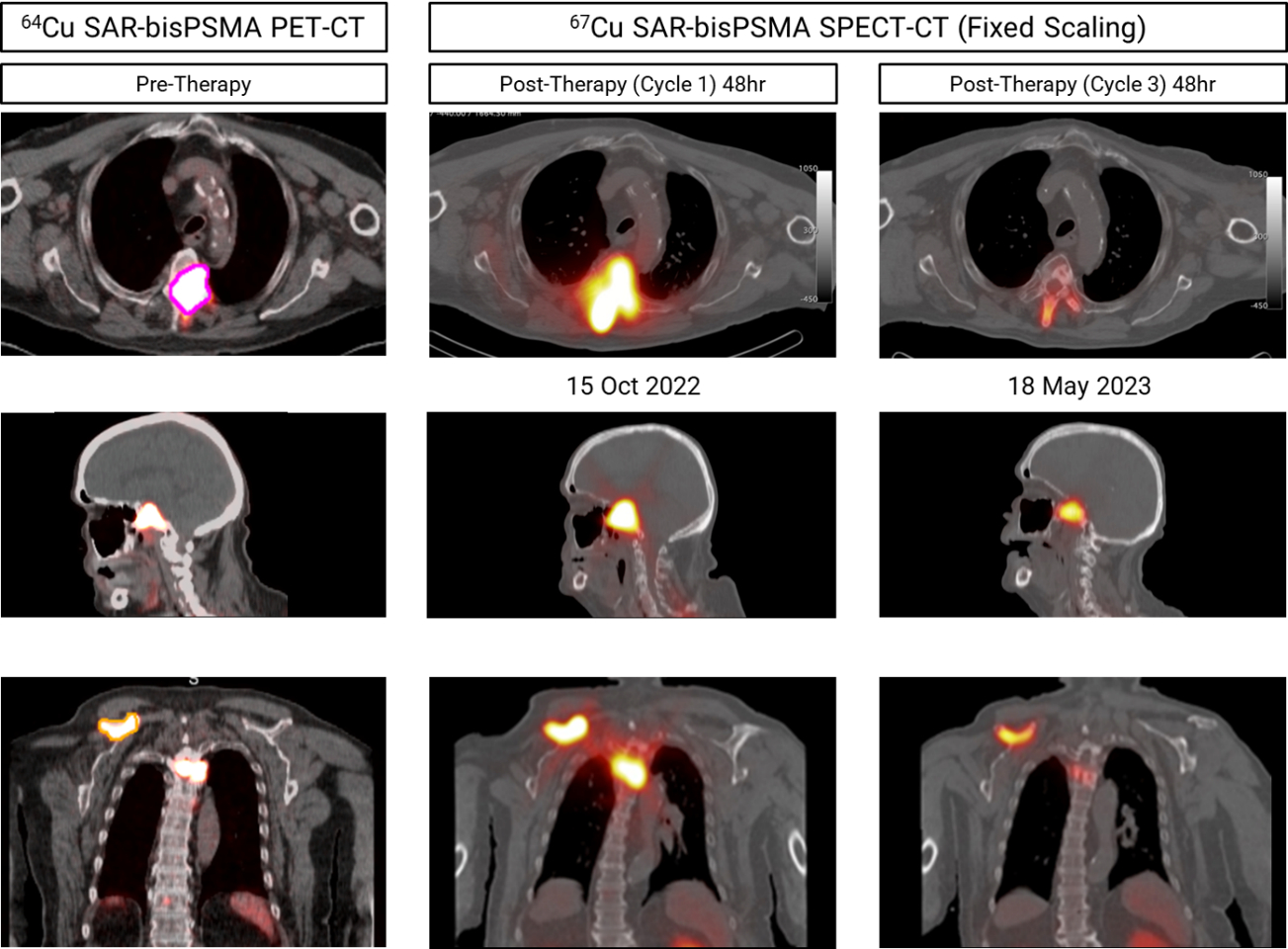
NorthStar Medical Radioisotopes, LLC (“NorthStar"), a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, and Clarity Pharmaceuticals (“Clarity”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, are pleased to announce the achievement of key milestones in the Cu-67 therapeutic radioisotope production program.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · June 26, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, today announced that it will be participating in the upcoming 2023 Guggenheim Radiopharmaceuticals Day. The event will be held in New York, NY, on May 15, 2023, and will include participating in an expert panel as well as 1x1 meetings with investors. Stephen Merrick, Chief Executive Officer of NorthStar, will be part of a panel discussion featuring leading companies focused on large-scale production of radioisotopes and CDMO suppliers, beginning at 12:00 p.m. ET.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · May 9, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, has announced the appointment of Jean-Noel David, MBA, MS, as Vice President, Operations. In this new position, he brings more than 20 years of operational and leadership expertise in the pharmaceutical and life sciences industry to the Company. Mr. David will have company-wide responsibility for Engineering, Manufacturing and Project Management, and report to President and Chief Operating Officer Frank Scholz, Ph.D.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · April 18, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, has announced the promotion of Jason Vinyard, MBA, MHA, to Vice President, Business Development, Therapeutic Technologies. Mr. Vinyard, who has been with NorthStar since 2021, was previously Senior Director, Business Development, Therapeutic Technologies, for the Company. He has more than 22 years of operational and commercial expertise in radioisotopes and radiopharmaceuticals. In this new position, Mr. Vinyard will have responsibility to advance the successful commercialization and market implementation of therapeutic radioisotopes, including copper-67 (Cu-67) and non-carrier added (n.c.a.) actinium-225 (Ac-225), and drive business development initiatives for NorthStar’s Radiopharmaceutical CDMO/CMO business units. He will report to NorthStar President and Chief Operating Officer Frank Scholz, Ph.D.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · April 12, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, today announced a corporate update highlighting progress across its key programs during the past twelve months, and indicating important upcoming milestones.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · April 3, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, today announced that it will be presenting at the upcoming Jefferies Radiopharma Innovation Summit. The event will be held in New York, NY, on April 3, 2023. Stephen Merrick, Chief Executive Officer of NorthStar, will present an overview of the Company from 8:00 a.m. - 8:30 a.m. ET, and participate in a panel discussion, “Deep Dive into Production of Alpha Emitters: Current and Future Directions,” from 12:15 - 1:00 p.m. ET.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · March 30, 2023
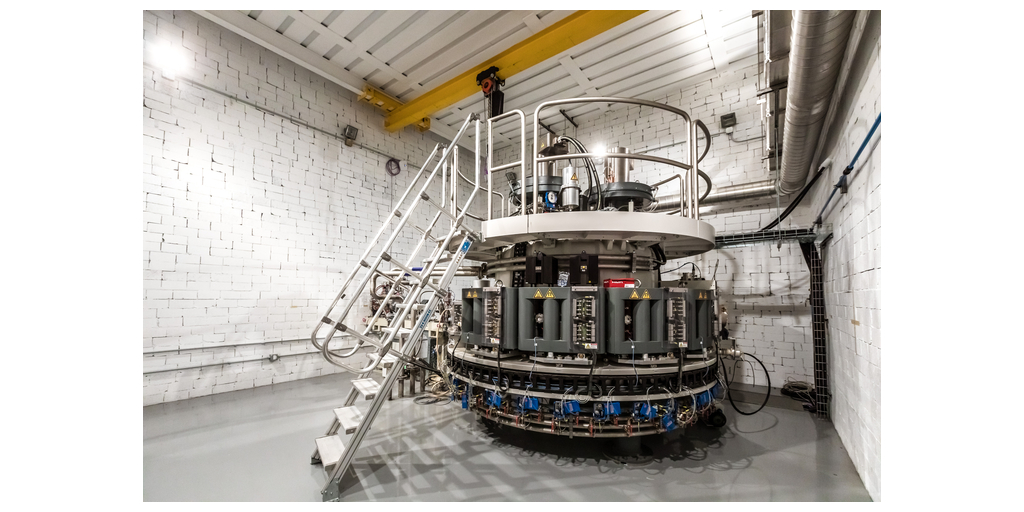
NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, announced that it has achieved a major milestone in its efforts to deliver commercial-scale production of the scarce therapeutic radioisotope actinium-225 (Ac-225). A custom-built IBA Rhodotron®TT 300-HE (High Energy) electron beam accelerator has been delivered to NorthStar’s new Ac-225 Production facility in Beloit, Wisconsin and installed in a specially designed building. This state-of-the-art facility will be dedicated exclusively to the production of non-carrier added (n.c.a.) Ac-225 and is part of NorthStar’s expansion plan to ensure scalable, reliable and environmentally preferable production of Ac-225 for treatment of patients with cancer and other serious diseases.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · March 1, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced it has achieved a major milestone in advancing its new technology for non-uranium based production of the critical medical radioisotope, molybdenum-99 (Mo-99). NorthStar’s proprietary electron accelerator technology has successfully produced Mo-99 at its recently completed Accelerator Production facility on its Beloit, Wis. campus. The “two beams on target” accelerator approach is highly efficient, with the potential to nearly double NorthStar’s commercial-scale Mo-99 capability with a single target set, and augments NorthStar’s ongoing domestic Mo-99 production in collaboration with the University of Missouri Research Reactor (MURR®). Like all NorthStar processes, it uses non-uranium based technology that is independent of reliance on overseas nuclear reactors. NorthStar’s technology produces far less radioactive waste than uranium-based Mo-99 production processes, which makes the NorthStar process more environmentally friendly. The U.S. Department of Energy’s National Nuclear Security Administration (DOE/NNSA) provided financial and technical support for the project as part of its program to increase U.S. production of the vital medical radioisotope Mo-99 without the use of highly enriched uranium, which is a proliferation-sensitive material. Mo-99 is the parent radioisotope of technetium-99m (Tc-99m), the most widely used diagnostic imaging radioisotope, used to inform healthcare decisions for over 40,000 U.S. patients daily.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · January 11, 2023

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced a significant expansion of its business platform with the formation of a new, patient-focused Radiopharmaceutical Contract Development and Manufacturing Organization (CDMO) services unit. Based on the success of its diagnostic imaging and therapeutic radioisotope production programs, NorthStar has created the services unit to further advance development and commercialization of radiopharmaceuticals to treat patients who have cancer and other serious diseases. The CDMO will provide collaborator companies with a full range of customized radiopharmaceutical development and commercialization services, and serve NorthStar in progressing its own radiopharmaceutical programs. Ground has been broken on a new facility, located alongside production facilities on the Company’s Beloit, Wis. campus. Upon completion, NorthStar will be the first and only U.S. company housing commercial-scale, multi-radioisotope production and radiopharmaceutical development services on the same campus, enabling collaborator companies to realize logistical, regulatory and cost benefits.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · November 15, 2022

Radiopharm Theranostics (ASX:RAD), a developer of a world-class platform of radiopharmaceutical products for both diagnostic and therapeutic uses, and NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced they have entered into a clinical supply agreement that will see NorthStar supply Radiopharm with Actinium-225.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · October 26, 2022

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and Eckert & Ziegler Isotope Technologies Dresden (ITD), a specialist for radiopharmaceutical plant engineering and fully owned subsidiary of Eckert & Ziegler AG (ISIN DE0005659700, SDAX), today announced an agreement for the purchase of hot cells and related equipment for NorthStar’s dedicated non-carrier-added (n.c.a.) actinium-225 (Ac-225) production facility. Hot cells are specially designed shielded enclosures that allow the safe handling of radioactive material. The equipment, worth several million USD, will be used by NorthStar in Beloit, Wisconsin, to produce commercial-scale quantities of n.c.a. Ac-225. Ac-225 is an emerging medical radioisotope for potential use in the treatment of cancer. The manufacturing process for these advanced radiopharmaceuticals requires innovative solutions and equipment to enable routine large-scale commercial production of Ac-225.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · October 11, 2022

NorthStar Medical Radioisotopes, LLC (‘NorthStar’), a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and IBA (Ion Beam Applications S.A., EURONEXT), the world leader in particle accelerator technology, today announced a new agreement under which NorthStar will purchase two additional Rhodotron® TT300 HE electron beam accelerators, and the associated beamlines, from IBA for the production of molybdenum-99 (Mo-99).
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · September 20, 2022

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced the signing of a long-term supply agreement for the therapeutic radioisotope actinium-225 (Ac-225) with Aktis Oncology, Inc. Under terms of the agreement, NorthStar will be a major supplier of high purity non-carrier-added (n.c.a.) Ac-225 to Aktis. Aktis will use NorthStar’s Ac-225 to advance development of its proprietary tumor-targeting agents intended to deliver transformative efficacy for patients with solid tumors.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · August 23, 2022

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced the signing of a long-term supply agreement with Clovis Oncology, Inc. (NASDAQ: CLVS) for the therapeutic medical radioisotope, actinium-225 (Ac-225). Under terms of the agreement, NorthStar will provide Clovis with its environmentally preferred, high purity non-carrier-added (n.c.a.) Ac-225. Clovis plans to use NorthStar’s Ac-225 to radiolabel its lead peptide-targeted radionuclide therapeutic candidate currently in development, FAP-2286, which targets fibroblast activation protein (FAP), a promising theranostic target with expression across many tumor types.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · July 19, 2022

NorthStar Medical Radioisotopes, LLC (‘NorthStar’), a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and Curie Therapeutics Inc., a therapeutics company dedicated to transforming cancer care with precision radiopharmaceuticals, today announced the signing of a long-term priority access supply agreement for the therapeutic medical radioisotope actinium-225 (Ac-225).
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · June 7, 2022

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, today announced the appointment of Monica Andersen as Vice President, Human Resources. In this new position, she will hold responsibility for the Company’s Human Resources and Organizational Health functions, reporting to NorthStar Executive Vice President and Chief Financial Officer, Paul Estrem. Ms. Andersen brings more than 20 years of senior human resources leadership, most recently as Vice President, Human Resources for Mallinckrodt Pharmaceuticals.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · May 24, 2022

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, today announced a corporate update highlighting progress across its key programs during the past twelve months, and upcoming milestones.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · March 29, 2022

NorthStar Medical Radioisotopes, LLC (‘NorthStar’), a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and IBA (Ion Beam Applications S.A., EURONEXT), the world leader in particle accelerator technology, today announced a new contract in which NorthStar will purchase a third Rhodotron® TT300 HE electron beam accelerator from IBA.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · November 18, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for medical imaging and therapeutic applications, hosted a growth and production expansion event, “Accelerating the Future of Patient Health,” at its headquarters in Beloit, Wis., on September 23, 2021. The event showcased the completion of NorthStar’s Accelerator Production and Isotope Processing facilities, which involved the installation of cutting-edge radioisotope production and processing equipment, and a groundbreaking celebration for its new state-of-the-art Therapeutic Radioisotope Production facility. Numerous guest speakers from national, state and local organizations joined with industry and healthcare leaders, NorthStar customers and investors to share perspectives on the exciting growth of nuclear medicine, the need for reliable U.S. radioisotope supply to support patient health and their experiences with NorthStar.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · October 14, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, is proud to announce the launch of its newly redesigned corporate website. The new site includes increased functionality and a number of enhancements. Expanded content includes latest product information, updates on NorthStar’s significant campus expansion, its growing partnerships and dynamic pipeline in therapeutic and SPECT radiopharmaceuticals, and the latest in career opportunities as the Company continues to grow and expand. NorthStar welcomes visitors to its new site at: www.northstarnm.com.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · October 7, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and POINT Biopharma Global Inc. (NASDAQ: PNT), a company accelerating the discovery, development, and global access to life changing radiopharmaceuticals, today announced the signing of a supply agreement for the therapeutic medical radioisotope actinium-225 (Ac-225).
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · September 8, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, today announced that Stephen Merrick, President and Chief Executive Officer of NorthStar Medical Radioisotopes, will present at the H.C. Wainwright 23rd Annual Global Investment Conference, being held in a virtual format September 13 – 15, 2021. Additionally, Company management will host one-on-one meetings during the conference.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · September 8, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for medical imaging and therapeutic applications, announced today that it has been awarded $37 million in cooperative agreement funds with the U.S. Department of Energy’s National Nuclear Security Administration (DOE/NNSA) as part of an industry outreach initiative to establish reliable domestic molybdenum-99 (Mo-99) production without the use of highly enriched uranium (HEU). NorthStar will use funds from the award to complete its neutron capture technology program and continue development and expansion of its accelerator production program. Both projects support non-uranium based, environmentally friendly production of the important medical radioisotope Mo-99. DOE/NNSA will provide $16.3M in funding for the neutron capture project and $20.7M for the accelerator project. NorthStar will also be required to provide an equal amount of matching funds. The awards will also be used in continuing development of enhancements for the FDA-approved and commercially available RadioGenix® System (technetium Tc-99m generator). The RadioGenix System uses reliable, domestic, non-uranium based Mo-99 to supply physicians and patients with technetium-99m (Tc-99m). Mo-99 is the parent radioisotope of Tc-99m, the most widely used medical imaging radioisotope, which is used to inform healthcare decisions for 40,000 U.S. patients daily.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · August 30, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for medical imaging and therapeutic applications, and GE Healthcare today announced the signing of an exclusive agreement for the manufacturing and distribution of iodine-123 (I-123) capsules in the United States. Under the contract terms, GE Healthcare’s Pharmaceutical Diagnostics unit will manufacture and supply NorthStar with I-123 capsules under the NorthStar label using a new, state-of-the-art production system at its facility in Arlington Heights, Ill. Upon receipt of the required regulatory approvals, NorthStar will retain exclusive U.S. marketing and distribution rights for these I-123 capsules, which will be available in 100µCi and 200µCi formulations.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · August 3, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for medical imaging and therapeutic applications, announced that it has achieved a major milestone in its efforts to expand U.S. production capacity for the important medical radioisotope, molybdenum-99 (Mo-99). The Company has received two custom-built IBA Rhodotron®TT 300-HE (High Energy) electron beam accelerators at its facility in Beloit, Wisconsin. The accelerators are critical components in a first-of-its-kind commercial-scale process to produce Mo-99, the parent radioisotope of technetium-99m, the most widely used medical imaging radioisotope, informing healthcare decisions for approximately 40,000 U.S. patients daily.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · May 11, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, today announced that Stephen Merrick, President and Chief Executive Officer of NorthStar Medical Radioisotopes, will present a corporate overview at the Emerging Medtech Summit 2021, on May 12, 2021 at 3:57 p.m. PT in Dana Point, Ca. The Emerging Medtech Summit 2021 brings together top strategists, investors and innovators driving the future of healthcare. Information about the Summit is available here.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · May 4, 2021

NorthStar Medical Radioisotopes, LLC, a global innovator in the development, production and commercialization of radiopharmaceuticals used for medical imaging and therapeutic applications, today announced a corporate update highlighting progress across its key programs during the past twelve months and upcoming milestones.
By NorthStar Medical Radioisotopes, LLC · Via Business Wire · April 13, 2021
