Articles from HistoSonics

HistoSonics, the developer of the Edison Histotripsy System, announced today 12-month follow-up clinical data from its prospective #HOPE4LIVER trials, designed to evaluate the safety and efficacy of histotripsy for the non-invasive destruction of primary (hepatocellular cancer-HCC) and metastatic liver tumors.
By HistoSonics · Via Business Wire · April 28, 2025

HistoSonics, the developer and manufacturer of the Edison® Histotripsy System, announced today the first patients with pancreatic tumors were successfully treated in the company sponsored GANNON trial. The feasibility trial is designed to evaluate the safety of histotripsy, a novel non-invasive technology that destroys targeted tumor tissue using focused ultrasound, in up to 30 patients with inoperable pancreatic adenocarcinoma tumors diagnosed with unresectable locally advanced disease (Stage 3) or those where a small number of tumors have spread to other parts of the body (Stage 4).
By HistoSonics · Via Business Wire · December 16, 2024

HistoSonics, the developer and manufacturer of the Edison® Histotripsy System, announced today the first patients were enrolled in their novel prospective master protocol study, called BOOMBOX, designed to evaluate the real-world use of histotripsy for treatment of liver tumors. BOOMBOX is a comprehensive, post-market master study that will provide important data for up to 5,000 patients treated across a growing global base of multi-disciplinary histotripsy providers. This master study design is unique in the medical device industry, as it captures treatment data across diverse tumor types and disease stages in patients with varying interventional needs, and amongst multiple clinical specialties.
By HistoSonics · Via Business Wire · November 18, 2024

HistoSonics, (www.histosonics.com), the developer and manufacturer of the Edison Histotripsy System, announced today they have been awarded an exclusive contract to provide Veteran’s Affairs hospitals across the United States access to their novel non-invasive tumor liquefying platforms. The contract, the work of the Veterans Health Administration (VHA) and the Strategic Acquisition Center (SAC) , establishes a $90 million ongoing schedule to develop histotripsy programs at key VA Hospitals. The announced VA contract is the culmination of a multi-year effort by HistoSonics, the company called Project Hero, to ensure meaningful value for Veterans and their families, VHA clinicians, administrators, and caregivers.
By HistoSonics · Via Business Wire · October 28, 2024

HistoSonics, (www.histosonics.com), the developer and manufacturer of the Edison® Histotripsy System, announced today the publication of the company’s pivotal clinical trials data in Radiology. The prospective, international, multi-center, single arm #HOPE4LIVER Trials were designed to evaluate the safety and efficacy of histotripsy for the non-invasive destruction of primary (hepatocellular carcinoma) and metastatic liver tumors. Safety and efficacy were measured against performance goals established using extensive real-world evidence from ablative therapies. The analysis included 49 targeted tumors in 44 patients: 18 with primary liver tumors and 26 with metastatic tumors from the colon, rectum, breast, pancreas, and other primary origins.
By HistoSonics · Via Business Wire · September 3, 2024

HistoSonics, (www.histosonics.com), the developer and manufacturer of the Edison™ Histotripsy System, announced today the company’s initial partnership in Asia with Hong Kong University receiving their first histotripsy system after a generous donation from the Li Ka Shing Foundation. The Foundation, led by its founder Mr. Li Ka-shing, is a prolific global philanthropic organization with a focused effort in Hong Kong, and is responsible to date for over hk$30 billion in projects involving education, medical services, charity, and anti-poverty programs. As part of the Foundation’s focus on bringing innovative medical advancements to Hong Kong, the Foundation provided the capital to acquire two initial histotripsy systems to the leading public hospitals in Hong Kong, one each to the University of Hong Kong and the Chinese University of Hong Kong. Chinese University of Hong Kong will receive the second histotripsy system early next year to complete the initial gift from Mr. Li.
By HistoSonics · Via Business Wire · August 28, 2024
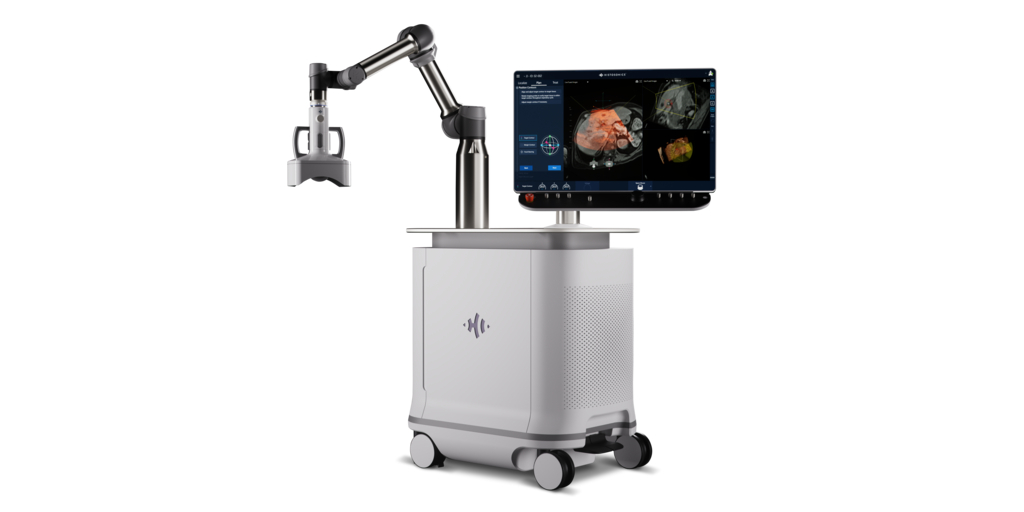
HistoSonics, the manufacturer of the Edison® Histotripsy System and novel histotripsy therapy platforms, announced today the completion of an oversubscribed $102 million Series D financing. The round was led by Alpha Wave Ventures, a world leader in growth stage investments, with participation from new investors Amzak Health and HealthQuest Capital, and existing investors Johnson & Johnson Innovation - JJDC, Inc. (JJDC), Venture Investors, Lumira Ventures, Yonjin Venture, the State of Wisconsin Investment Board, and others.
By HistoSonics · Via Business Wire · August 15, 2024

HistoSonics®, (www.histosonics.com), the manufacturer of the Edison® System and novel histotripsy therapy platforms, announced today that the company’s Edison System has been selected to participate in the UK’s newly created Innovative Devices Access Pathway (IDAP) Pilot Program designed to accelerate the development of cost-effective medical devices and their integration into the UK market. Applications to compete for one of the 8 available places in the highly sought after UK program began in 2023 and was open to UK and international commercial and non-commercial developers with new health technology solutions.
By HistoSonics · Via Business Wire · February 14, 2024
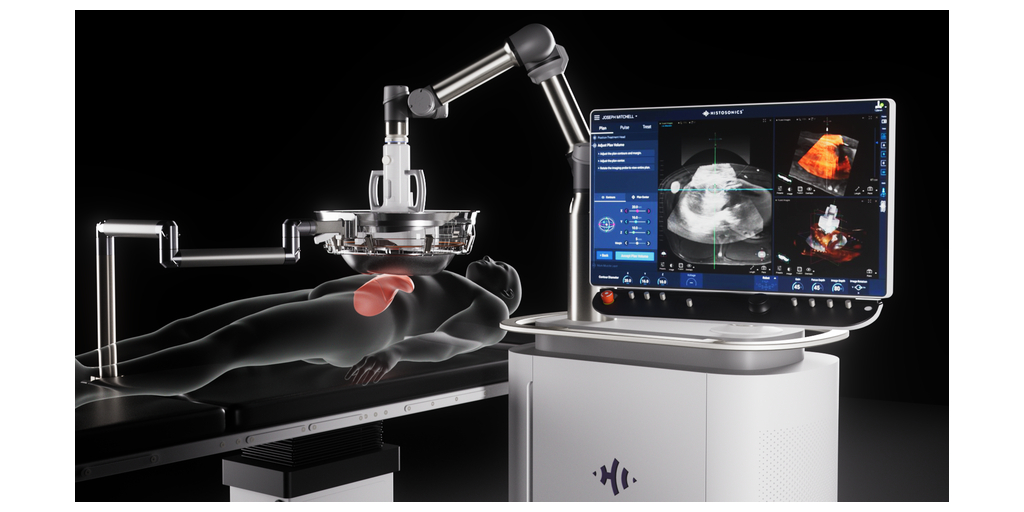
HistoSonics, (www.histosonics.com), the manufacturer of the non-invasive Edison® Histotripsy System, announced today a patient from the University of Rochester Medical Center has received the world’s first targeted liver tumor treatments utilizing the Edison System following its recent FDA De Novo clearance. In the same week Cleveland Clinic treated their initial patients suffering from liver tumors utilizing histotripsy. HistoSonics' image guided sonic beam therapy system uses proprietary technology and advanced imaging to deliver non-invasive, personalized treatments with precision and control. The science of histotripsy uses focused sound energy to produce controlled acoustic cavitation that mechanically destroys and liquifies targeted tissue at sub-cellular levels.
By HistoSonics · Via Business Wire · January 5, 2024
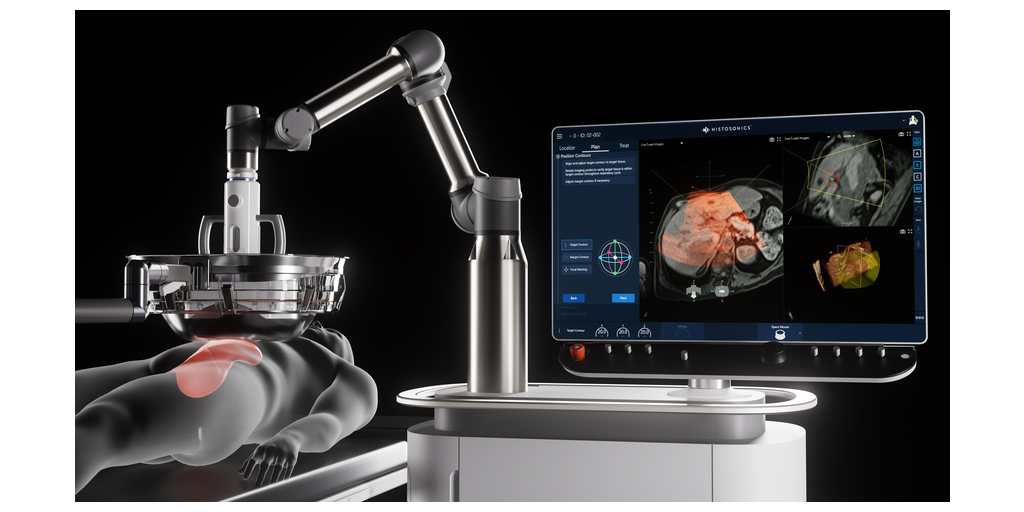
HistoSonics, (www.histosonics.com), the manufacturer of the non-invasive Edison® Histotripsy System and novel sonic beam therapy, announced today that the Centers of Medicare & Medicaid Services (CMS) set a new payment rate for histotripsy of liver tumors procedures, increasing the payment rate to $17,500. In their November 2023 rule making process, CMS utilized claims data from the #HOPE4LIVER Trial conducted in the United States to increase the outpatient payment rate for HistoSonics Category III Current Procedural Terminology (CPT®) Code 0686T, the non-thermal ablation via acoustic energy delivery of malignant hepatocellular tissue including image guidance, from $12,500 to the new national average rate of $17,500, assigning the novel procedure to Ambulatory Payment Classification (APC) 1576. This payment rate supports hospitals and physicians using the non-invasive histotripsy procedure in the outpatient setting to destroy targeted liver tumors while existing Diagnosis Related Group (DRG) payment rates are applicable for patients who require hospital admission.
By HistoSonics · Via Business Wire · November 6, 2023
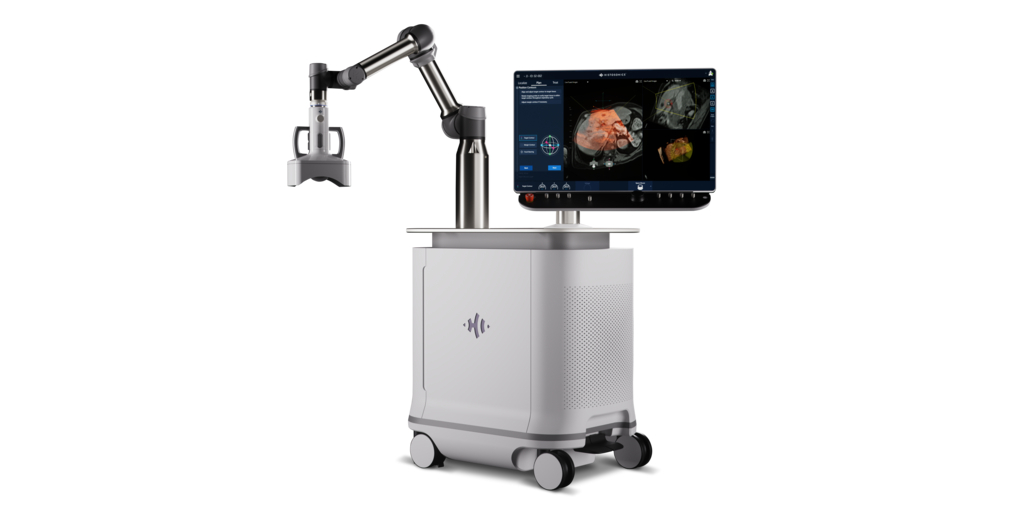
HistoSonics®, (www.histosonics.com), the manufacturer of the Edison® System and novel histotripsy therapy platforms, announced today the marketing authorization of its “Breakthrough” platform via the U.S. Food and Drug Administration's (FDA) De Novo Classification Request process, a rigorous pre-market review pathway for medical devices with no existing predicate. Marketing authorization makes Edison the first and only histotripsy platform available in the Unites States.
By HistoSonics · Via Business Wire · October 9, 2023
