Articles from Alto Neuroscience, Inc.

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced the publication of a review, "Brain Neuroplasticity Mechanisms in Psychiatric Illnesses and in the Development of Novel Treatments," in the American Journal of Psychiatry. The review was co-authored by members of Alto Neuroscience’s leadership team, Patricio O’Donnell, M.D., Ph.D, and Amit Etkin, M.D., Ph.D., as well as Husseini Manji, M.D., former global head of therapeutics, neuroscience at Janssen and a member of Alto’s board of directors.
By Alto Neuroscience, Inc. · Via Business Wire · February 3, 2026

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced the issuance of U.S. Patent Number 12,521,374 covering methods of treating depression with ALTO-207, a novel combination of pramipexole and ondansetron. The patent protects a key aspect of ALTO-207 – the use of ondansetron to mitigate pramipexole-related side effects so that patients can achieve the higher dose levels needed to realize the antidepressant benefits of pramipexole. The newly issued patent claims further strengthen the company’s growing intellectual property estate supporting ALTO-207, a differentiated investigational therapy being developed for patients with treatment-resistant depression.
By Alto Neuroscience, Inc. · Via Business Wire · January 14, 2026

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced multiple presentations highlighting new data and analyses supporting the Company’s development programs at the 64th annual meeting of the American College of Neuropsychopharmacology (ACNP) held January 12-15, 2026.
By Alto Neuroscience, Inc. · Via Business Wire · January 13, 2026

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that on December 2, 2025, the Compensation and Management Development Committee of Alto’s Board of Directors granted a new employee an option to purchase 150,000 shares of Alto’s common stock. The stock option was granted under Alto’s 2025 Inducement Plan as a material inducement to the individual entering employment with Alto in accordance with NYSE Listed Company Manual Rule 303A.08.
By Alto Neuroscience, Inc. · Via Business Wire · December 3, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today reported financial results for the quarter ended September 30, 2025, and highlighted recent progress across its pipeline of clinical-stage product candidates.
By Alto Neuroscience, Inc. · Via Business Wire · November 12, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that members of the Company’s management team will present at the following upcoming investor conferences:
By Alto Neuroscience, Inc. · Via Business Wire · October 28, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, announced today that it has entered into a securities purchase agreement with institutional and accredited investors to sell securities in a private placement financing (the “PIPE”) for gross proceeds of approximately $50 million, before deducting offering expenses. The financing was led by Perceptive Advisors, with participation by new and existing institutional investors, including Commodore Capital, Vestal Point Capital, Vivo Capital, and a large biotech dedicated investor.
By Alto Neuroscience, Inc. · Via Business Wire · October 20, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, announced today that, following a successful outcome from a recent FDA meeting, it plans to accelerate the development of ALTO-207 for people with treatment resistant depression (TRD). The $50 million private placement announced today supports expanded development of ALTO-207, and the Company expects to initiate a Phase 3 study by early 2027 following Phase 3 readiness work and alignment with the FDA on the planned study design.
By Alto Neuroscience, Inc. · Via Business Wire · October 20, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to ALTO-101 for the treatment of cognitive impairment associated with schizophrenia (CIAS). There are currently no approved treatments for CIAS, a core feature of schizophrenia that severely impacts daily functioning and quality of life for millions of patients.
By Alto Neuroscience, Inc. · Via Business Wire · October 3, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced positive results from an independent, prospective replication study evaluating electroencephalography (EEG) biomarkers in people with schizophrenia. The study successfully replicated previous findings, demonstrating that event-related theta-band responses, particularly theta-band inter-trial coherence (ITC), robustly differentiate people with schizophrenia from healthy individuals.
By Alto Neuroscience, Inc. · Via Business Wire · September 9, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that members of the Company management team will present at the following upcoming investor conferences and events:
By Alto Neuroscience, Inc. · Via Business Wire · August 18, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today reported financial results for the quarter ended June 30, 2025, and highlighted recent progress across its pipeline of clinical-stage product candidates.
By Alto Neuroscience, Inc. · Via Business Wire · August 13, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced the appointment of Raymond Sanchez, M.D., to its Board of Directors, effective August 12, 2025. Dr. Sanchez is a highly accomplished executive with a strong background in medicine and over 20 years of strategic experience in the life sciences industries.
By Alto Neuroscience, Inc. · Via Business Wire · August 13, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today highlighted The Lancet Psychiatry publication of data from the PAX-D study evaluating pramipexole in patients with treatment-resistant depression (TRD). The study was conducted by the University of Oxford and was funded by the UK government’s National Institute for Health and Care Research. Results showed pramipexole augmentation of antidepressant treatment, at a target dose of 2.5mg, demonstrated a large (Cohen’s d=0.87) reduction in symptoms relative to placebo at 12 weeks, but was associated with a high rate of adverse effects. The link to the online publication can be found here. The PAX-D study results guided Alto’s acquisition of ALTO-207, a fixed-dose combination of pramipexole and the antiemetic, ondansetron.
By Alto Neuroscience, Inc. · Via Business Wire · June 30, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced the identification of a patient selection biomarker and positive pharmacodynamic results from its exploratory Phase 2 proof-of-concept (POC) trial of ALTO-203 in major depressive disorder (MDD) patients with elevated levels of anhedonia. ALTO-203 is a novel, oral, histamine H3 inverse agonist, designed to modulate circuits underlying cognition, wakefulness, and alertness.
By Alto Neuroscience, Inc. · Via Business Wire · June 26, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that it entered into an asset purchase agreement with Chase Therapeutics Corporation for a portfolio of potentially best-in-class dopamine agonist drug combinations, including ALTO-207, formerly known as CTC-501, for treatment resistant depression (TRD), generally defined as a failure on two or more antidepressants.
By Alto Neuroscience, Inc. · Via Business Wire · June 3, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced a presentation at the American Society of Clinical Psychopharmacology (ASCP) Annual Meeting, in Scottsdale, Arizona, held May 27-30, 2025.
By Alto Neuroscience, Inc. · Via Business Wire · May 29, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that members of the Company management team will present at the following upcoming investor conferences and events in June:
By Alto Neuroscience, Inc. · Via Business Wire · May 28, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today reported financial results for the first quarter ended March 31, 2025, and highlighted recent progress across its pipeline of clinical-stage product candidates.
By Alto Neuroscience, Inc. · Via Business Wire · May 14, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced multiple presentations at the Society of Biological Psychiatry (SOBP) Annual Meeting, in Toronto, Canada, held April 24-26, 2025.
By Alto Neuroscience, Inc. · Via Business Wire · April 28, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that members of the Company management team will present at the JonesResearch Virtual CNS Day on Tuesday, April 29, 2025, at 12:30 pm ET and participate in one-on-one investor meetings.
By Alto Neuroscience, Inc. · Via Business Wire · April 22, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today reported financial results for the full-year ended December 31, 2024, and highlighted recent progress across its pipeline of clinical-stage product candidates.
By Alto Neuroscience, Inc. · Via Business Wire · March 20, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that members of the Company management team will present at the following upcoming investor conferences and events in March:
By Alto Neuroscience, Inc. · Via Business Wire · February 25, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that the U.S. Patent and Trademark Office has granted U.S. Patent Number 12,226,375, with method claims pertaining to treatment of major depressive disorder (MDD) in patients with inadequate response to an antidepressant. The method includes treatment with ALTO-300 as an adjunctive therapy and patient selection using specific electroencephalogram (EEG) measures. As granted, not accounting for any potential patent term extensions, the patent is expected to cover the use of ALTO-300 in the EEG-defined patient population until 2044.
By Alto Neuroscience, Inc. · Via Business Wire · February 19, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced a favorable outcome from the planned interim analysis for the Phase 2b trial of ALTO-300 as an adjunctive treatment for patients with major depressive disorder (MDD). Based on the results of the interim analysis, the Phase 2b trial will continue with an increase of approximately 50 biomarker positive patients in the final analysis sample. Topline results are expected in mid-2026.
By Alto Neuroscience, Inc. · Via Business Wire · February 12, 2025

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced multiple presentations at the 63rd annual meeting of the American College of Neuropsychopharmacology (ACNP), in Phoenix, Arizona, held December 8-11, 2024.
By Alto Neuroscience, Inc. · Via Business Wire · December 11, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today reported financial results for the third quarter ended September 30, 2024, and highlighted recent corporate progress.
By Alto Neuroscience, Inc. · Via Business Wire · November 12, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that members of the Company management team will present at the following upcoming investor conferences and events in November:
By Alto Neuroscience, Inc. · Via Business Wire · November 4, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that the Phase 2b study of ALTO-100 in patients with major depressive disorder (MDD) did not meet its primary endpoint, assessed by a change from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS), compared to placebo. The favorable safety and tolerability profile of ALTO-100 was consistent with previously reported studies.
By Alto Neuroscience, Inc. · Via Business Wire · October 22, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced a peer-review publication demonstrating baseline cognitive performance is not a moderator of response to standard-of-care antidepressants in patients with major depressive disorder (MDD). The analysis, titled “Baseline Cognition Is Not Associated With Depression Outcomes in Vortioxetine for Major Depressive Disorder: Findings From Placebo-Controlled Trials,” was published online in The Journal of Clinical Psychiatry.
By Alto Neuroscience, Inc. · Via Business Wire · September 4, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that members of the Company management team will present at the following upcoming investor conferences and events in September:
By Alto Neuroscience, Inc. · Via Business Wire · September 3, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today reported financial results for the second quarter ended June 30, 2024, and highlighted recent corporate progress.
By Alto Neuroscience, Inc. · Via Business Wire · August 13, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced that the Company has been awarded $11.7 million by the Wellcome Trust to advance the clinical development of lead candidate ALTO-100 through a Phase 2b study in patients with bipolar depression characterized by a cognitive biomarker. The Company has initiated this study and expects to report topline data in 2026.
By Alto Neuroscience, Inc. · Via Business Wire · July 25, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO), a clinical-stage biopharmaceutical company focused on the development of novel precision medicines for neuropsychiatric disorders, today announced the completion of enrollment in its Phase 2b study of ALTO-100 in adults with major depressive disorder (MDD). Topline results from this study are expected to be reported in October 2024.
By Alto Neuroscience, Inc. · Via Business Wire · July 16, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced the initiation of a Phase 2 double-blind, placebo-controlled study of its transdermal formulation of ALTO-101, a novel PDE4 inhibitor in development for the treatment of cognitive impairment associated with schizophrenia (CIAS). Alto expects to report top-line data from the Phase 2 study in the second half of 2025.
By Alto Neuroscience, Inc. · Via Business Wire · June 20, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced that Amit Etkin, M.D., Ph.D., founder and CEO, was named an Entrepreneur Of The Year® 2024 Bay Area Award finalist by EY. Now in its 38th year, Entrepreneur Of The Year is the preeminent competitive business award for audacious leaders who disrupt markets, revolutionize sectors, and have a transformational impact on lives. Over the past four decades, the program has recognized daring entrepreneurs with big ideas and bold actions that reshape our world, across all industries and sectors.
By Alto Neuroscience, Inc. · Via Business Wire · June 18, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced that Company management will participate in the Jefferies Global Healthcare Conference, taking place June 5-6, 2024, in New York. Amit Etkin, M.D., Ph.D., founder and chief executive officer, will participate in a fireside chat on Thursday, June 6, 2024, at 2:30 p.m. ET, and management will host one-on-one meetings with investors.
By Alto Neuroscience, Inc. · Via Business Wire · May 29, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced the expansion of the Company’s leadership team with the addition of industry veteran Michael Hanley as chief operating officer (COO). Mr. Hanley brings over twenty-five years of leadership experience in the life sciences industry, with established expertise across corporate operations, product development, strategy, and commercialization, with relevant expertise in CNS/neuroscience-focused companies. As Alto’s COO, Mike will be responsible for new product planning, portfolio strategy, and cross-functional leadership to maximize the impact of the Company’s clinical-stage programs and biomarker platform.
By Alto Neuroscience, Inc. · Via Business Wire · May 21, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today reported financial results for the first quarter ended March 31, 2024, and highlighted recent corporate progress.
By Alto Neuroscience, Inc. · Via Business Wire · May 14, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced upcoming data presentations that highlight the company’s precision psychiatry pipeline and biomarker-based analyses at the Society of Biological Psychiatry (SOBP) and American Society of Clinical Psychopharmacology (ASCP) Annual Meetings, to take place May 9-11 in Austin, TX, and May 28-31 in Miami Beach, FL, respectively.
By Alto Neuroscience, Inc. · Via Business Wire · May 9, 2024
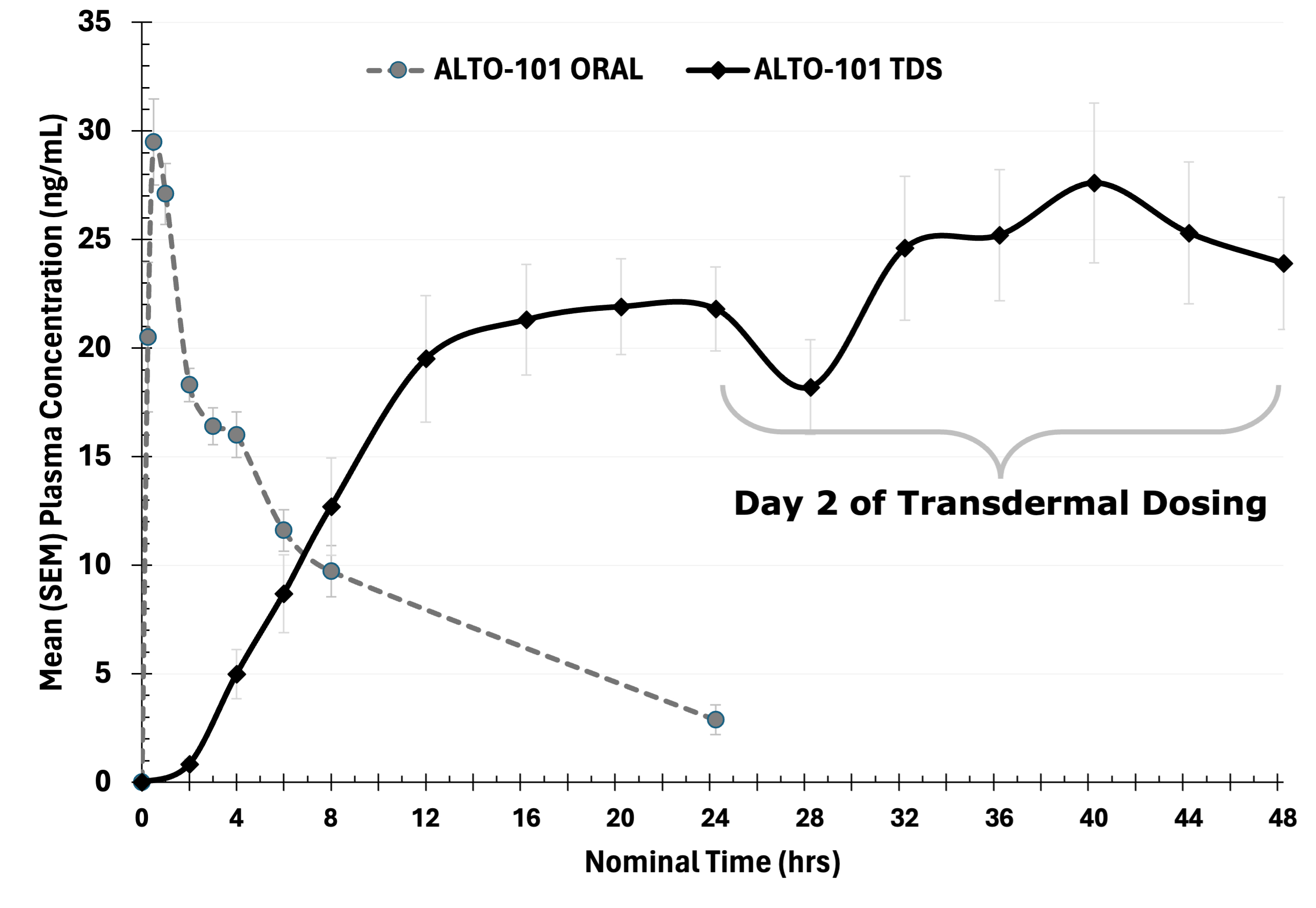
Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced positive results from its healthy volunteer Phase 1 study of ALTO-101, a novel PDE4 inhibitor in development for cognitive impairment associated with schizophrenia (CIAS). Results from the study demonstrated favorable tolerability and improved pharmacokinetics of ALTO-101 when administered via a transdermal delivery system (TDS) compared to oral administration. The TDS formulation is being developed in partnership with MEDRx. The drug exposure levels achieved with the transdermal formulation were significantly greater than systemic exposure with oral administration while also reducing typical class-wide adverse events. The Company plans to initiate a proof-of-concept study evaluating ALTO-101 in patients with CIAS in the first half of 2024, with topline data expected in the second half of 2025.
By Alto Neuroscience, Inc. · Via Business Wire · April 23, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced the initiation of its Phase 2 double-blind, single- and multiple-dose study to determine the pharmacodynamic effects of ALTO-203 in MDD patients as well as assess its safety, tolerability, and pharmacokinetics. To date, ALTO-203 has demonstrated positive emotional and cognitive effects in healthy participants after a single dose. The present study will evaluate these effects in patients with MDD to determine the potential of ALTO-203 as an antidepressant.
By Alto Neuroscience, Inc. · Via Business Wire · April 3, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today reported financial results for the full year ended December 31, 2023, and highlighted recent corporate progress.
By Alto Neuroscience, Inc. · Via Business Wire · March 21, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced the appointment of Maha Radhakrishnan, M.D. to the company’s board of directors. Dr. Radhakrishnan is an accomplished industry executive with decades of experience advancing large strategic portfolios across different therapeutic areas through product development and commercialization and overseeing the global medical strategy and operations within major biotechnology and pharmaceutical companies.
By Alto Neuroscience, Inc. · Via Business Wire · March 11, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced that company management will participate in the following upcoming investor conferences:
By Alto Neuroscience, Inc. · Via Business Wire · March 7, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced that Amit Etkin, M.D., Ph.D., founder and chief executive officer, will present a company overview at the TD Cowen 44th Annual Healthcare Conference on Tuesday, March 5, 2024, at 1:30 pm ET.
By Alto Neuroscience, Inc. · Via Business Wire · February 27, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced the closing of its previously announced upsized initial public offering of 9,246,000 shares of common stock, which includes the exercise in full by the underwriters of their option to purchase 1,206,000 additional shares, at a public offering price of $16.00 per share. The aggregate gross proceeds to Alto from the offering were approximately $147.9 million before deducting underwriting discounts and commissions and other offering expenses payable by Alto. All of the shares of common stock were offered by Alto. Alto’s common stock is listed on the New York Stock Exchange under the ticker symbol “ANRO.”
By Alto Neuroscience, Inc. · Via Business Wire · February 6, 2024

Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced the pricing of its upsized initial public offering of 8,040,000 shares of common stock at a public offering price of $16.00 per share. The aggregate gross proceeds to Alto from the offering are expected to be approximately $128.6 million before deducting underwriting discounts and commissions and other offering expenses payable by Alto. In addition, Alto has granted the underwriters a 30-day option to purchase up to an additional 1,206,000 shares of common stock at the initial public offering price, less underwriting discounts and commissions. All of the shares of common stock are being offered by Alto.
By Alto Neuroscience, Inc. · Via Business Wire · February 1, 2024

Alto Neuroscience, Inc. today announced details from three presentations on clinical development programs presented at the 62nd annual meeting of the American College of Neuropsychopharmacology (ACNP) that took place in Tampa, Florida, from December 3-6, 2023.
By Alto Neuroscience, Inc. · Via Business Wire · December 7, 2023

Alto Neuroscience, Inc. today announced positive results from its Phase 2a study of ALTO-300 at the 62nd Annual Meeting of the American College of Neuropsychopharmacology (ACNP), demonstrating clinically meaningful improvements and favorable safety and tolerability in patients with major depressive disorder (MDD). Following administration of ALTO-300, patients characterized by an electroencephalogram (EEG) biomarker demonstrated robust clinical improvement in depression symptoms and higher response rates, as measured by the Montgomery–Åsberg Depression Rating Scale (MADRS), compared to patients without the EEG biomarker. These results support the potential of ALTO-300 as a novel treatment for MDD. The company has initiated a Phase 2b study evaluating ALTO-300 in 200 patients with MDD, which is expected to read out in the first half of 2025.
By Alto Neuroscience, Inc. · Via Business Wire · December 4, 2023
